Has your steering wheel been too hot to touch this summer? A new thermoelectric material reported in the journal Science could offer relief. The widespread adoption of thermoelectric devices... Read more
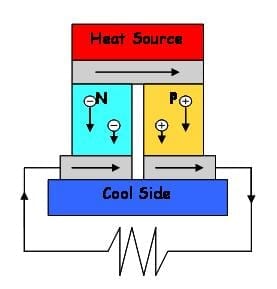
Technology developed at MIT can harness temperature fluctuations of many kinds to produce electricity. Thermoelectric devices, which can generate power when one side of the device is a diffe... Read more
One strategy for addressing the world’s energy crisis is to stop wasting so much energy when producing and using it One strategy for addressing the world’s energy crisis is to stop wasting s... Read more
Its figure of merit (a rating for thermoelectric efficiency) is one of the highest ever recorded for a bulk material Thermoelectric materials work by converting differences in temperature in... Read more