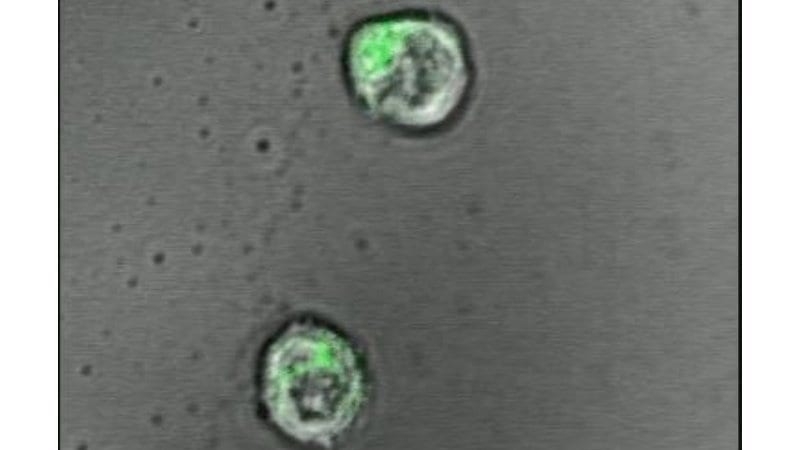
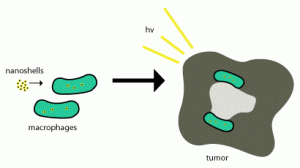
Successful T cell engineering with gene scissors The idea of genetically modifying a patient’s own immune cells and deploying them against infections and tumors has been around since the 198... Read more
In a proof-of-principle study in mice, scientists at Johns Hopkins Medicine report the creation of a specialized gel that acts like a lymph node to successfully activate and multiply cancer-... Read more
Loyola University Medical Center has launched the first clinical trial in the Midwest of an experimental melanoma treatment that genetically engineers a patient’s immune system to figh... Read more
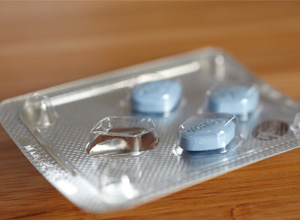
A clinical breakthrough might be on the cards as a German study has suggested that the impotence drug may be the most effective skin cancer treatment, reports Forbes. A clinical breakthrough... Read more
Vaccines work by exposing the body to an infectious agent in order to prime the immune system to respond quickly when it encounters the pathogen again. Some vaccines, such as the diphtheria... Read more
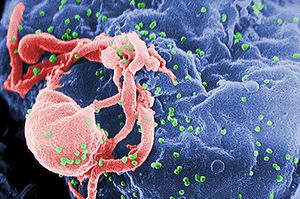
Image via Wikipedia Could also be used against a range of chronic viral diseases A proof-of-principle study has demonstrated that it is possible to engineer human blood stem cells into cells... Read more