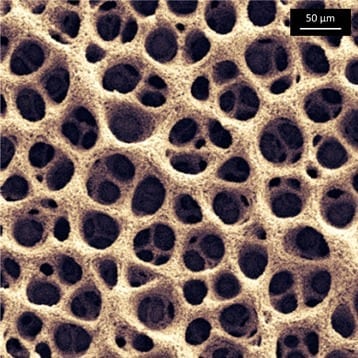
Micro-supercapacitors are a promising alternative to micro-batteries because of their high power and long lifetime. They have been in development for about a decade but until now they have s... Read more
A new method for “recycling” hydrogen-containing fuel materials could open the door to economically viable Read more
A cunning plan to store energy underwater may help fulfil the promise of wind power THE problem with wind power is that is cannot always be relied upon. The wind—and other transient, environ... Read more