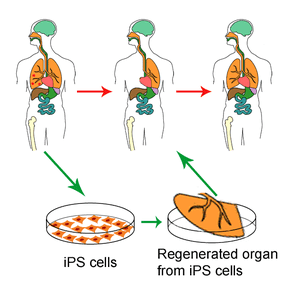
Researchers have generated functional hepatocytes from human stem cells, transplanted them into mice with acute liver injury, and shown the ability of these stem-cell derived human liver cel... Read more
It would not take an elaborate plot by Al Qaeda to endanger many lives. SINCE Sept. 11, 2001, the American government, under two presidents, has taken unprecedented steps to ensure the safet... Read more