Scientists are working diligently to prepare for the expected increase in global population — and therefore an increased need for food production— in the coming decades. A team of engineers... Read more
Researchers at Lancaster University are using X-rays to help farmers increase yields and cut water pollution following an unexpected discovery in a pea and bean crop. Plant and Soil Scientis... Read more
New data and assessments suggest that resilience of the planet is now at risk Almost half of the processes that are crucial to maintaining the stability of the planet have become dangerously... Read more
Hazardous substances, such as toxic heavy metals, can also be removed relatively easily with magnets Phosphorus can be found in fertilizers, drinks and detergents. It accumulates in waterway... Read more
Lakes and streams are often receiving so much phosphorous that it could pose a threat to the local aquatic environment. Now, research from the University of Southern Denmark shows that there... Read more
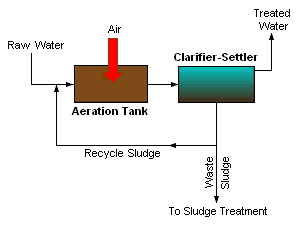
Sewage sludge, wastewater and liquid manure are valuable sources of fertilizer for food production. Fraunhofer researchers have now developed a chemical-free, eco-friendly process that enabl... Read more
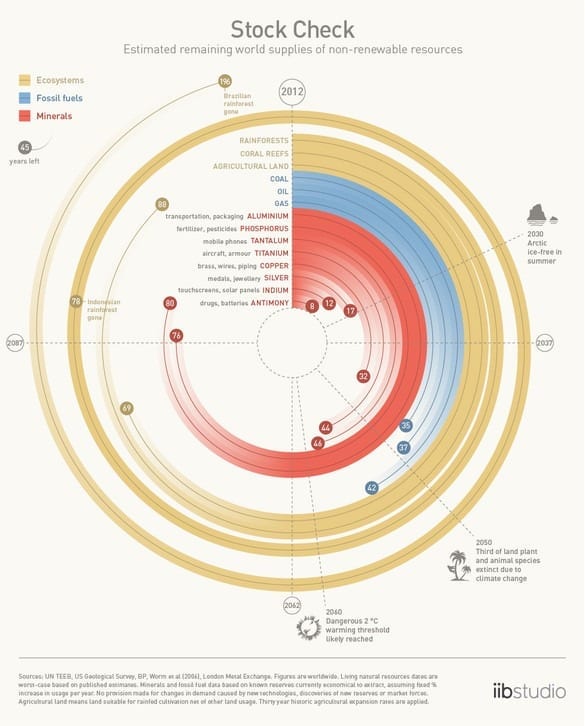
This graphic shows you how long we have to reach peak everything–from oil to phosphorus. We use a lot of materials that we can’t get back once they’re gone. Much ado has been made abou... Read more
Image via Wikipedia IN the far reaches of Shaanxi Province in northern China, in an apple-producing village named Ganquanfang, I recently visited a house belonging to two cheery primary-scho... Read more