Mini midbrains provide next generation platforms to investigate human brain biology, diseases and therapeutics Scientists in Singapore have made a big leap on research on the ‘mini-brain’. T... Read more
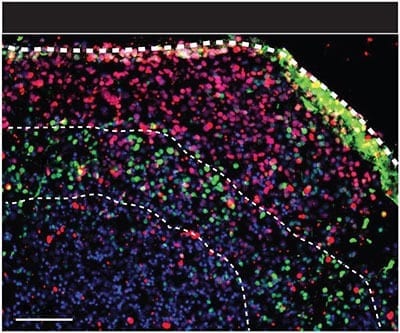
Networking neurons thrive in 3-D human “organoid” A patient tormented by suicidal thoughts gives his psychiatrist a few strands of his hair. She derives stem cells from them to grow budding... Read more
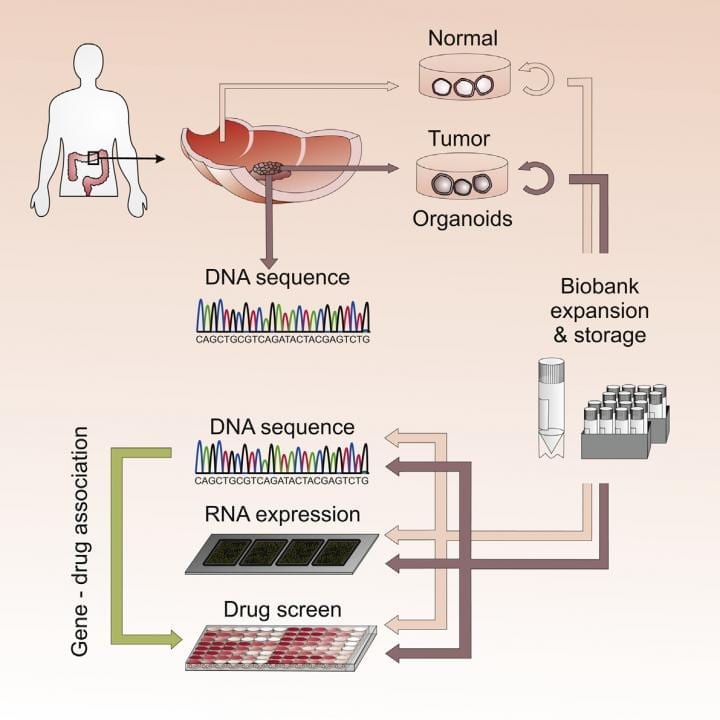
Three-dimensional cultures (or “organoids”) derived from the tumors of cancer patients closely replicate key properties of the original tumors, reveals a study published May 7 in... Read more