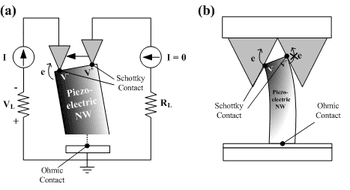
Charging mobile phones with sound, like chants from at football ground, could become a reality, according to a new collaboration between scientists from Queen Mary University of London and N... Read more
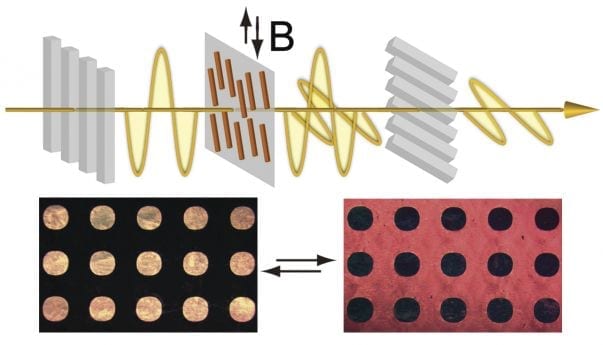
The discovery has applications in signage, posters, writing tablets, billboards and anti-counterfeit technology Chemists at the University of California, Riverside have constructed liquid cr... Read more
A color-coded smart tag could tell consumers whether a carton of milk has turned sour or a can of green beans has spoiled without opening the containers, according to researchers. The tag, w... Read more
Researchers have designed tiny, light-controlled gold particles that can release DNA controls to switch blood clotting off and on. The results are reported July 24 in the open access journal... Read more
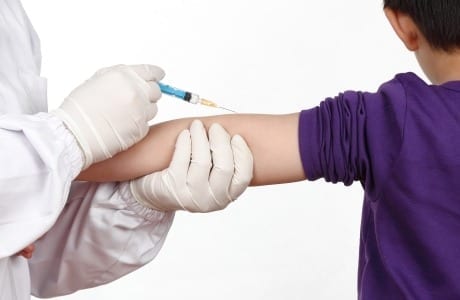
Scientists in the US have developed a novel vaccination method that uses tiny gold particles to mimic a virus and carry specific proteins to the body’s specialist immune cells. The technique... Read more
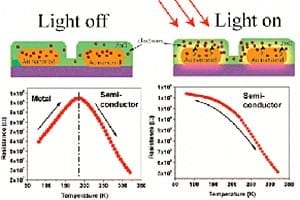
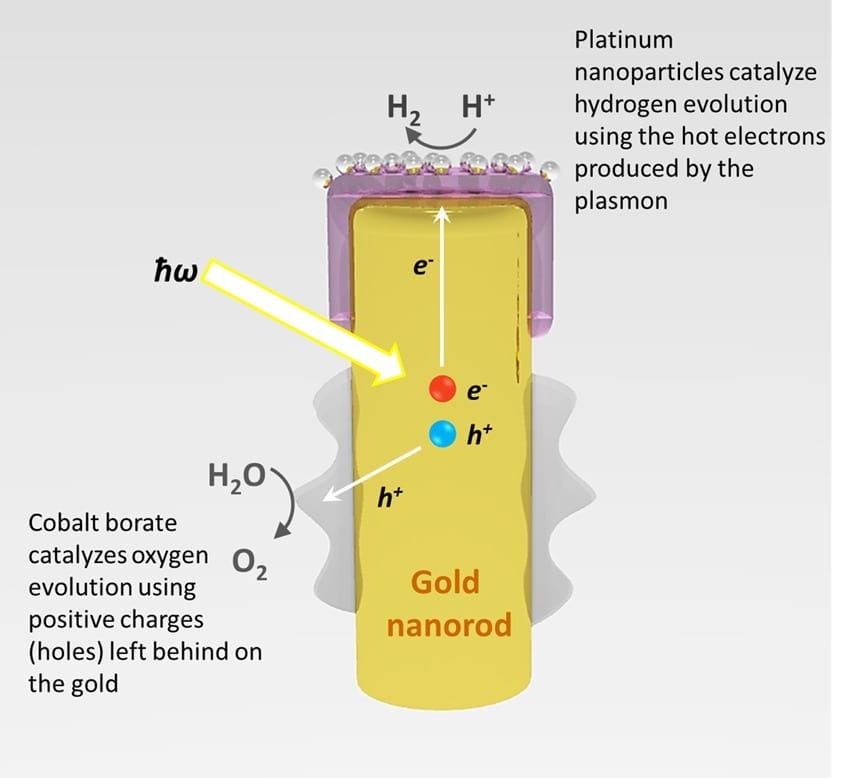
More than 60% of the investment in making microbial fuel cells is the cost of platinum Engineers at the University of Wisconsin-Milwaukee (UWM) have identified a catalyst that provides the s... Read more
What do fireflies, nanorods, and Christmas lights have in common? Someday, consumers may be able to purchase multicolor strings of light that don’t need electricity or batteries to glo... Read more
Chemists at the University of California, Riverside, are developing a future display technology using nanoscale-sized iron oxide rods that shine when exposed to an external magnetic field. T... Read more