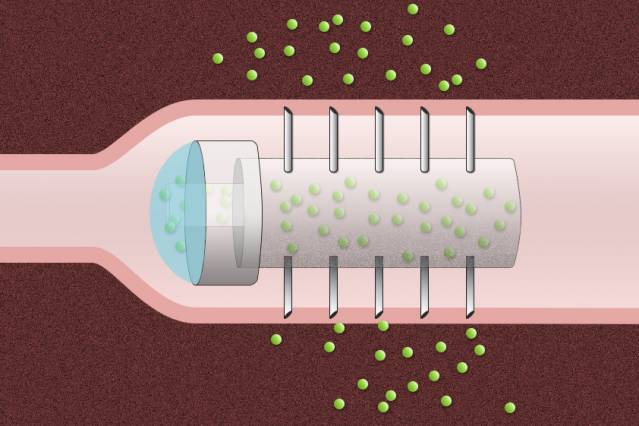
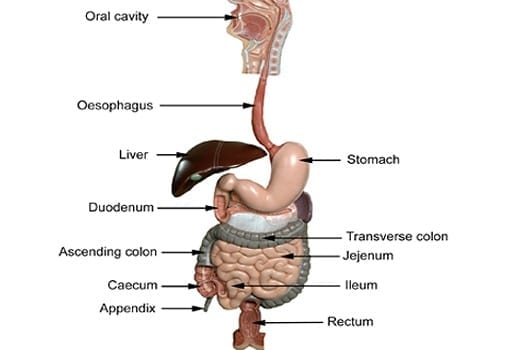
Given a choice, most patients would prefer to take a drug orally instead of getting an injection. Unfortunately, many drugs, especially those made from large proteins, cannot be given as a p... Read more
A BREAKTHROUGH in drug testing developed by a University of Huddersfield lecturer could lead to cheaper, more effective medicines. Dr Hamid Merchant is a member of the team that has create... Read more
Colonoscopies can be an uncomfortable procedure for patients who may already be worried about what results will be found. When the results are inconclusive, a patient’s options can be... Read more
VARIANT OF COMMON SOIL-BASED PATHOGEN FOUND FOR THE FIRST TIME IN A PATIENT WITH MS RESEARCHERS FIND EVIDENCE OF SIMILAR INFECTION IN OTHER MS PATIENTS A research team from Weill Cornell Med... Read more
Other research shows treatment leads to high patient satisfaction Swallowing pills containing a concentrate of fecal bacteria successfully stops recurrent bouts of debilitating Clostridium d... Read more
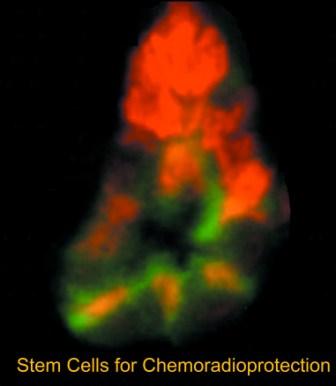
Treating a cancerous tumor is like watering a houseplant with a fire hose—too much water kills the plant, just as too much chemotherapy and radiation kills the patient before it kills the tu... Read more
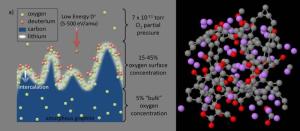
The study of the lithium coatings also impacts many areas beyond magnetic fusion, including nanoelectronics, lithium batteries, computational materials science, bioengineering and biophysics... Read more