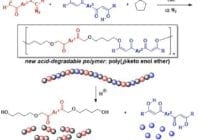
A research team in Ehime University prepared a new type of synthetic polymer, which can be degraded into a combination of well-defined low molecular weight compounds under very mild acidic c... Read more
Rich countries prospered without worrying much about the environment. Poor and middle-income countries do not have that luxury ON THE southern shore of Lake Naivasha, Kenya’s lush Rift Valle... Read more
The future of global agriculture Trying to tap into the best thinking about the future of global agriculture, as I have tried to do in my work as a reporter, can be an exercise in frustratio... Read more
It might sound like fighting fire with fire, but geologist Chen Zhu proposes the application of another industrial waste to the Hungarian bauxite residue spill, with the aim of reducing toxi... Read more