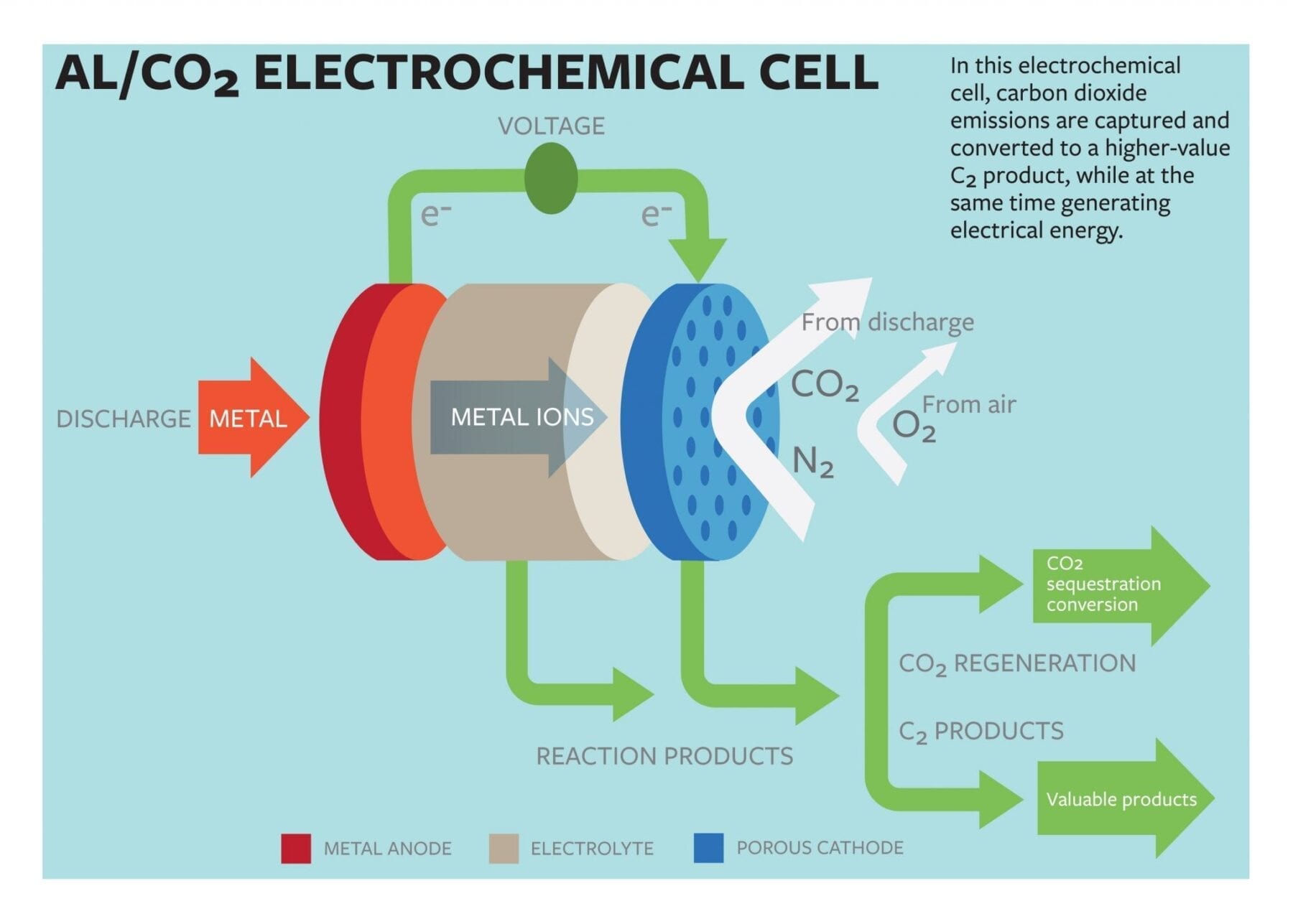
While the human race will always leave its carbon footprint on the Earth, it must continue to find ways to lessen the impact of its fossil fuel consumption. “Carbon capture” technologies – c... Read more
A University of Texas at Arlington materials science and engineering team has developed a new energy cell that can store large-scale solar energy even when it’s dark. The innovation is an ad... Read more
Image via Wikipedia With the use of the new super material graphene, Swedish and American researchers have succeeded in producing a new type of lighting component. It is inexpensive to produ... Read more
Image via Wikipedia Various efforts are underway to find a cheap, efficient and scalable way to recycle the greenhouse gas carbon dioxide back into the hydrocarbons that fuel civilization In... Read more