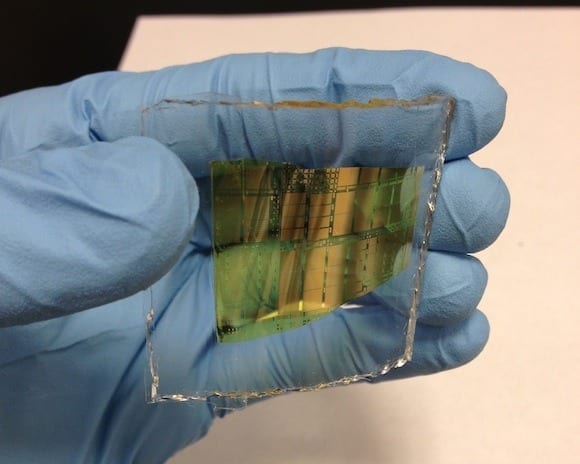
When it comes to electronics, silicon will now have to share the spotlight. In a paper recently published in Nature Communications, researchers from the USC Viterbi School of Engineering des... Read more
Nanoengineers at the University of California, San Diego have developed a 3D-printed device inspired by the liver to remove dangerous toxins from the blood. The device, which is designed to... Read more
A new generation of potentially safer and more cost-effective therapeutics against West Nile virus, and other pathogens An international research group led by Arizona State University profes... Read more
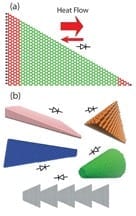
Researchers are proposing a new technology that might control the flow of heat the way electronic devices control electrical current, an advance that could have applications in a diverse ran... Read more
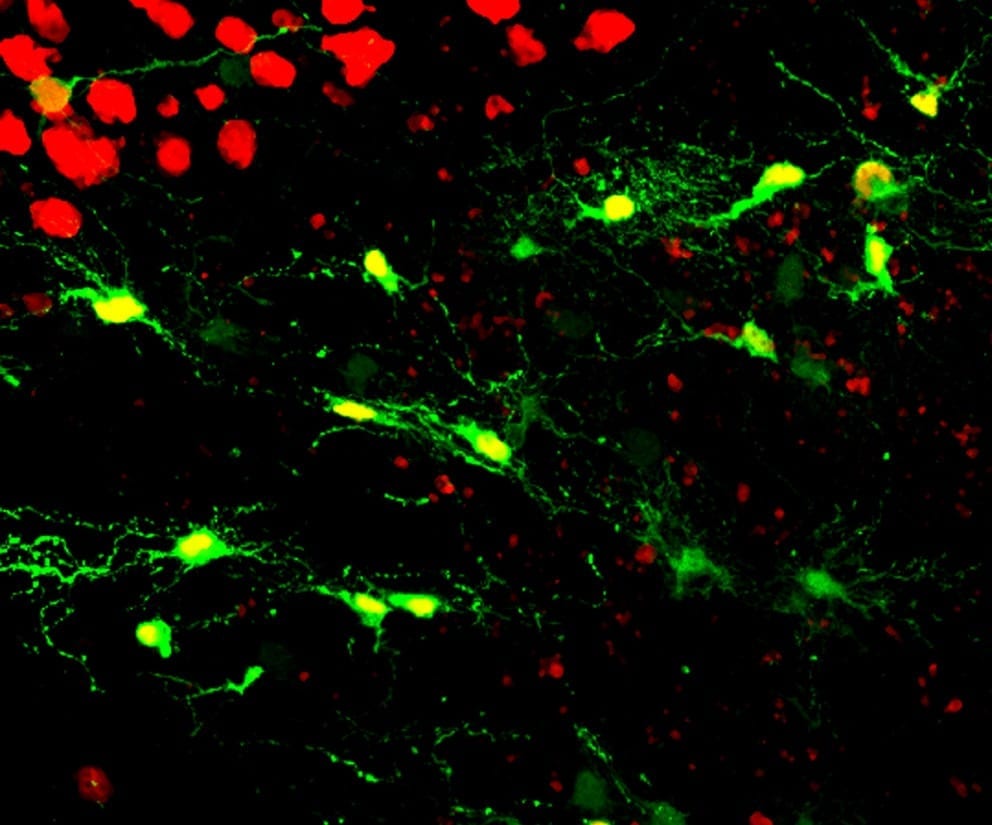
Researchers at Penn State have developed an innovative technology to regenerate functional neurons after brain injury and also in model systems used for research on Alzheimer’s disease... Read more
Running Buddies Enrichment Program provides additional incentives for joggers to exercise by pairing them up with a dog that’s waiting to be adopted. The program is connected with the Stray... Read more
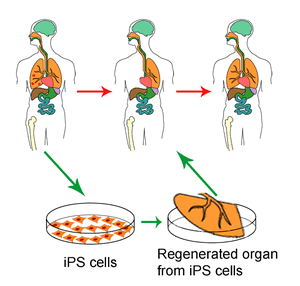
Can we actually reverse aging? A new study led by researchers at the University of California, Berkeley, represents a major advance in the understanding of the molecular mechanisms behind ag... Read more
“Our test will improve the accuracy of diagnosis, leading to the proper treatment of patients affected by WNV,” While the United States has largely been spared the scourge of mosquito-borne... Read more
The goal is to be able to print biological tissues for regenerative medicine. Nanoengineers at the University of California, San Diego have developed a novel technology that can fabricate, i... Read more
Contour Crafting concept technology could change the way we construct buildings. Professor Behrokh Khoshnevis from the University of Southern California, has come up with a concept that allo... Read more