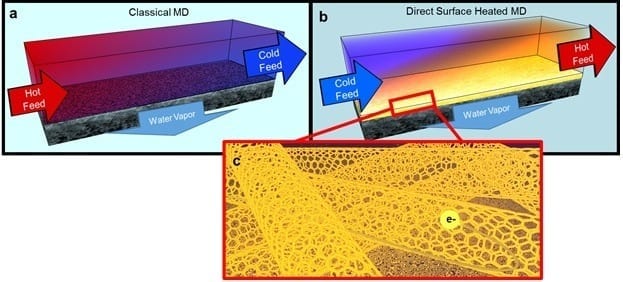
UCR research expands efforts to provide clean water for the world’s growing population Engineers at the University of California, Riverside have developed a new way to recover almost 100 per... Read more
The world needs clean water The world needs clean water, and more and more, we’re pulling it from the oceans, desalinating it, and drinking it. But what to do with the salty bri... Read more
It might sound like fighting fire with fire, but geologist Chen Zhu proposes the application of another industrial waste to the Hungarian bauxite residue spill, with the aim of reducing toxi... Read more