UNSW scientists have developed an efficient oxygen-producing electrode for splitting water that can be scaled up to produce hydrogen – a clean energy fuel
SYDNEY chemists may have removed one of the main hurdles to the “hydrogen economy” after developing what is believed to be the world’s most efficient water-splitting electrode.
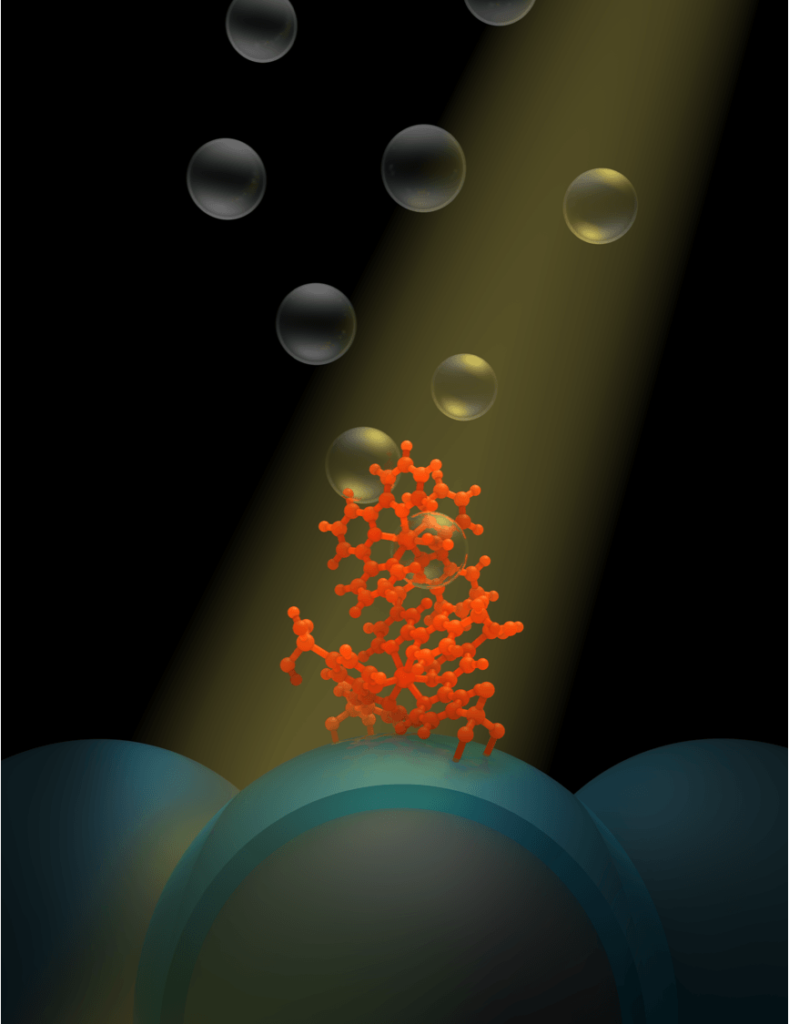
The conductor, outlined overnight in an article in the journal Nature Communications, is made entirely of nickel and iron. Its inventors say it outperforms state-of-the-art electrodes based on iridium and ruthenium — metals so rare that only a few dozen tonnes are produced globally each year.
The article’s co-author, Chuan Zhao, said the cost and sustainability of these elements was a big impediment to industrial-scale electrolysis of water.
“Our electrode is made entirely of non-precious metals,” said Dr Zhao, of the University of NSW.
“I don’t see why this cannot be used in industry. There are no major hurdles to producing this electrode and using it in an industrial electrolyser.”
Hydrogen has long been considered preferable to carbon-based fuel sources because it produces water when it burns. Theoretically, it can fuel cars and power stations, heat homes and store excess electricity without emitting toxins or warming the atmosphere.
But hydrogen does not exist naturally in its pure form and the cleanest way of producing it — by splitting water into its constituent elements of hydrogen and water — is extremely expensive and energy-intensive.
The new approach, which Dr Zhao described as a proof of concept, addresses both problems by using inexpensive elements and reducing the amount of electricity needed.
Read more: Hydrogen economy a step closer with water-splitting breakthrough
The Latest on: Hydrogen economy
[google_news title=”” keyword=”Hydrogen economy” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Hydrogen economy
- With China’s EV sector at top speed, Beijing eyes ‘economical’ hydrogen-power vehicles amid green energy transitionon April 19, 2024 at 2:30 pm
China is the world’s top producer and consumer of electric vehicles, and Beijing is now seeking the lead in hydrogen-powered vehicles.
- Hungarian Group Inaugurates Green Hydrogen Production Projecton April 19, 2024 at 10:50 am
A Hungarian company that operates refineries and petrochemical plants, and runs service stations across Central and Eastern Europe, said it is set to ...
- Green Hydrogen Summit sets out roadmap to future fuels economyon April 19, 2024 at 4:56 am
Abu Dhabi, UAE: Abu Dhabi Future Energy Company PJSC – Masdar, the UAE’s clean energy powerhouse, has hailed the conclusion of a highly successful Green Hydrogen Summit focused on accelerating the ...
- Colombia eyes US$700mn investments in green hydrogen, digital economy: Trade Ministeron April 19, 2024 at 4:53 am
Germán Umaña Mendoza, Minister of Trade, Industry, and Tourism of Colombia, said that his country, under the framework of the Comprehensive Economic Partnership Agreement (CEPA) with the UAE, aims to ...
- Hydrogen may disappear from EPA’s power plant rule. Here’s what that means.on April 19, 2024 at 3:24 am
The agency will likely choose a different technology to underpin pollution standards for new "intermediate" natural gas plants.
- Coal transition: Betting on hydrogen with an energy technology granton April 19, 2024 at 2:30 am
Lewis County Transit got a $1.8 million grant from the coal transition fund to install a micro-sized hydrogen electrolyzer, which will use electricity to split water molecules into hydrogen and oxygen ...
- SA to establish a Green Hydrogen Skills Centre to plug the skills gapon April 18, 2024 at 12:26 pm
CHIETA, the Mining Qualification Authority(MQA), and Transport Education Training Authority (TETA) will collaborate to establish this multimillion-dollar centre by next year.
- South Africa faces skills gap in hydrogen economy, report findson April 17, 2024 at 1:41 pm
The Labour Market Intelligence report has found that occupational workers lack hydrogen-specific skills to compete in the global economy ...
- The pros and pitfalls of hydrogen power, according to MIT energy experton April 17, 2024 at 9:35 am
Even with these drawbacks, Stoner said the benefit of hydrogen is that it is super energy dense and can be a substitute in industries that currently depend on fossil fuels. Hydrogen has nearly three ...
- The Top 3 Hydrogen Stocks Leading the Green Energy Revolutionon April 16, 2024 at 12:56 pm
InvestorPlace - Stock Market News, Stock Advice & Trading Tips The road to net zero has pushed hydrogen into the spotlight as a clean energy ...
via Bing News