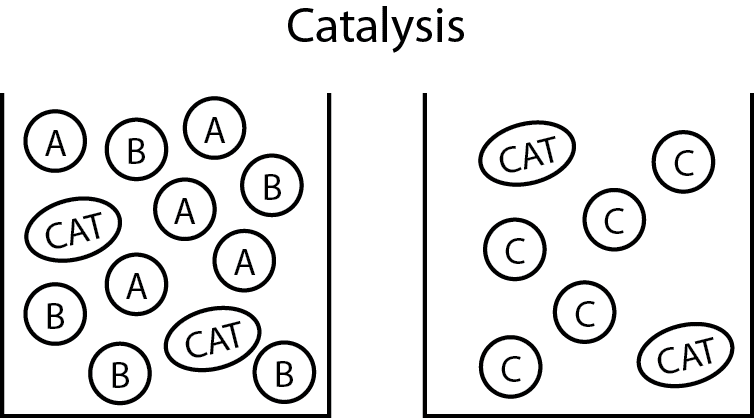
English: This figure shows that the catalyst, although integral to the reaction, does not react into the product. Because of this some catalysts can be recovered. Other catalysts, like those used in the manufacture of polyethylene, are used in such a small quantity they are left in the product. (Photo credit: Wikipedia)
LATEST NEWS
- The Site is finally back and stable. Big changes in the wind . . . soon! Thanks for your patience and support!
Copyright 2024. Created by Innovation Toronto