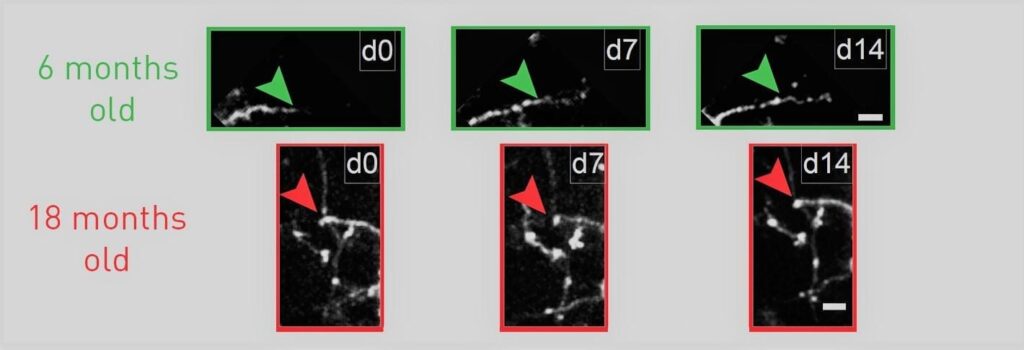
Tracking the growth of neuron arbors under the microscope over two weeks, researchers typically saw growth beyond the arrow marker in six-month-old mice (green), but none beyond the marker in 18-month old mice (red).
A new study provides fresh evidence that the decline in the capacity of brain cells to change, called “plasticity,” rather than a decline in total cell number may underlie some of the sensory and cognitive declines associated with normal brain aging. Scientists at MIT’s Picower Institute for Learning and memory show that inhibitory interneurons in the visual cortex of mice remain just as abundant during aging, but their arbors become simplified and they become much less structurally dynamic and flexible.
In their experiments published online in the Journal of Neuroscience they also show that they could restore a significant degree of lost plasticity to the cells by giving treating mice with the commonly used antidepressant medication fluoxetine, also known as Prozac.
“Despite common belief, loss of neurons due to cell death is quite limited during normal aging and unlikely to account for age-related functional impairments,” wrote the scientists, including lead author Ronen Eavri and corresponding author Elly Nedivi, a professor of biology and brain and cognitive sciences. “Rather it seems that structural alterations in neuronal morphology and synaptic connections are features most consistently correlated with brain age, and may be considered as the potential physical basis for the age-related decline.”
Learn more: Antidepressant restores youthful flexibility to aging inhibitory neurons in mice
The Latest on: Reversing cognitive decline
[google_news title=”” keyword=”reversing cognitive decline” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Reversing cognitive decline
- America Is in Decline | Opinionon April 19, 2024 at 7:01 am
Is America's downturn merely another dip in a long arc of non-linear, yet essentially upward, progress? Or is it, rather, the first phase of steep and irreversible national decline?
- Thought Provoking Work May Reduce Later Life Cognitive Declineon April 17, 2024 at 1:30 pm
Engaging in complex, thought-provoking work may lower the risk of mild cognitive impairment (MCI) in older age.
- Routine jobs raise the risk of cognitive decline by 66% and dementia by 37%, study sayson April 17, 2024 at 1:00 pm
People who have cognitively demanding jobs are much less likely to experience cognitive decline and dementia in their 70s, a study finds.
- Can Weight Loss Surgery, Medications, and Lifestyle Changes Improve Brain Health?on April 15, 2024 at 11:11 am
Surgery, medications, and lifestyle changes can all help a person lose weight. But can they also improve brain health? Here's what to know.
- Step steady: Consistent walking improves brain function in older adultson April 8, 2024 at 10:25 pm
Study highlights that stabilizing daily step variability, rather than increasing total step counts, improves cognitive flexibility in older adults following a 10-week physical activity intervention.
- Cognitive decline may be detected using network analysis, according to Concordia researcherson April 8, 2024 at 5:00 pm
Most people would be correct to laugh it off as a normal part of aging. But for others, cognitive decline may start as a worrying but clinically unnoticeable step toward cognitive impairment, be it ...
- Reversing the decline among M40on April 8, 2024 at 5:00 pm
PETALING JAYA: More than 600, 000 households from the middle 40% (M40) income group have slipped into the bottom 40% (B40) category as the Covid-19 crisis delivered a major blow on Malaysians ...
- Cognitive decline may be detected using network analysis, according to researcherson April 8, 2024 at 5:00 pm
Most people would be correct to laugh it off as a normal part of aging. But for others, cognitive decline may start as a worrying but clinically unnoticeable step toward cognitive impairment ...
- Cognitive Decline Not Associated With Occasional Adolescent Cannabis Useon April 7, 2024 at 5:00 pm
The research ultimately confirmed that subjects who occasionally used cannabis exhibited no significant changes in cognitive functioning compared to their non-cannabis-using peers. Researchers ...
- Obesity and a high fat diet may accelerate brain aging, lead to cognitive declineon April 6, 2024 at 8:17 am
Obesity and a high-fat diet may damage blood vessels in the brain and accelerate aging, a new study in mice suggests.
via Bing News