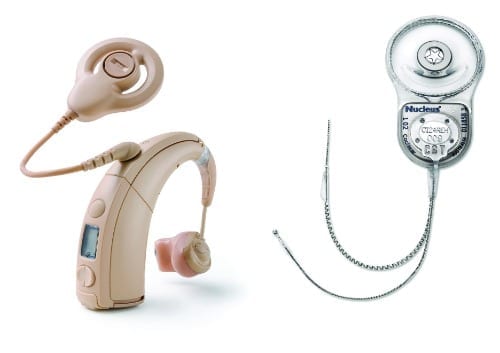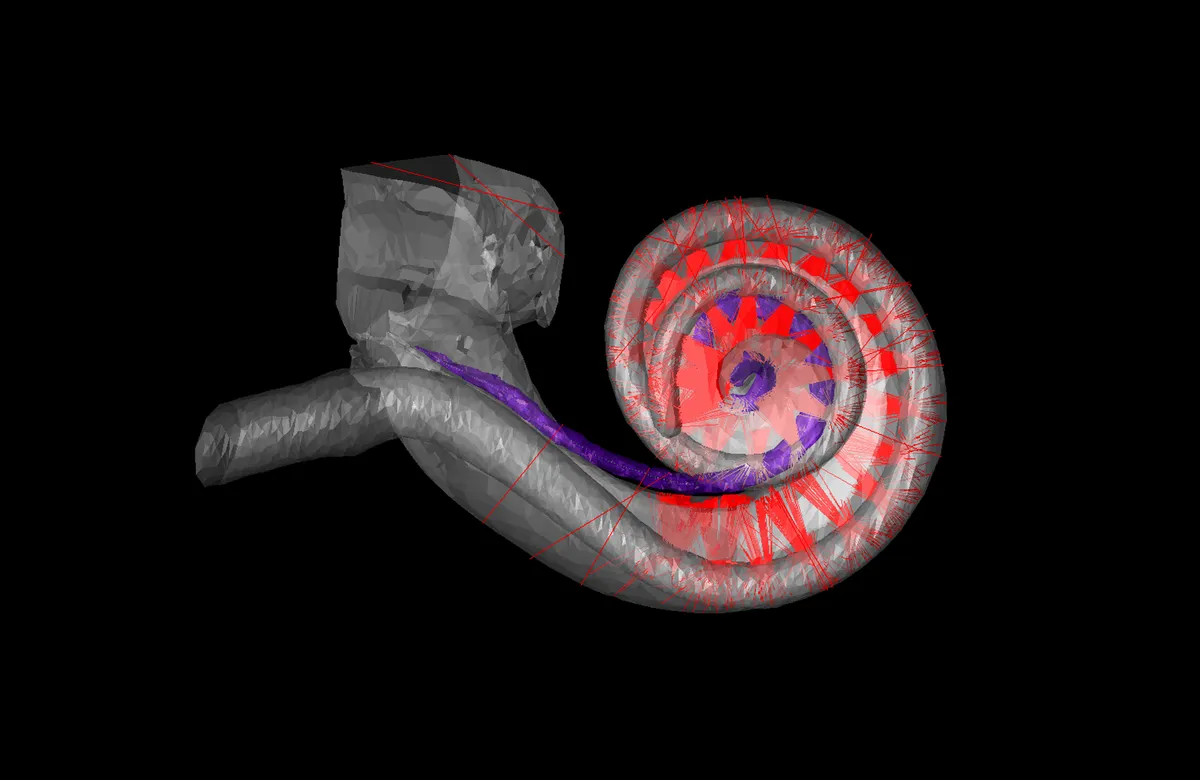
Human hearing depends on the cochlea, a snail-shaped structure in the inner ear. A new kind of cochlear implant for people with disabling hearing loss would use beams of light to stimulate the cochlear nerve.
LAKSHAY KHURANA AND DANIEL KEPPELER
HERE’S A POPULAR misconception that cochlear implants restore natural hearing. In fact, these marvels of engineering give people a new kind of “electric hearing” that they must learn how to use.
Natural hearing results from vibrations hitting tiny structures called hair cells within the cochlea in the inner ear. A cochlear implant bypasses the damaged or dysfunctional parts of the ear and uses electrodes to directly stimulate the cochlear nerve, which sends signals to the brain. When my hearing-impaired patients have their cochlear implants turned on for the first time, they often report that voices sound flat and robotic and that background noises blur together and drown out voices. Although users can have many sessions with technicians to “tune” and adjust their implants’ settings to make sounds more pleasant and helpful, there’s a limit to what can be achieved with today’s technology.
I have been an otolaryngologist for more than two decades. My patients tell me they want more natural sound, more enjoyment of music, and most of all, better comprehension of speech, particularly in settings with background noise—the so-called cocktail party problem. For 15 years, my team at the University of Göttingen, in Germany, has been collaborating with colleagues at the University of Freiburg and beyond to reinvent the cochlear implant in a strikingly counterintuitive way: using light.
We recognize that today’s cochlear implants run up against hard limits of engineering and human physiology. So we’re developing a new kind of cochlear implant that uses light emitters and genetically altered cells that respond to light. By using precise beams of light instead of electrical current to stimulate the cochlear nerve, we expect our optical cochlear implants to better replicate the full spectral nature of sounds and better mimic natural hearing. We aim to start clinical trials in 2026 and, if all goes well, we could get regulatory approval for our device at the beginning of the next decade. Then, people all over the world could begin to hear the light.
How cochlear implants work
Some 466 million people worldwide suffer from disabling hearing loss that requires intervention, according to the World Health Organization. Hearing loss mainly results from damage to the cochlea caused by disease, noise, or age and, so far, there is no cure. Hearing can be partially restored by hearing aids, which essentially provide an amplified version of the sound to the remaining sensory hair cells of the cochlea. Profoundly hearing-impaired people benefit more from cochlear implants, which, as mentioned above, skip over dysfunctional or lost hair cells and directly stimulate the cochlear, or auditory, nerve.
In the 2030s, people all over the world could begin to hear the light.
Today’s cochlear implants are the most successful neuroprosthetic to date. The first device was approved by the U.S. Food and Drug Administration in the 1980s, and nearly 737,000 devices had been implanted globally by 2019. Yet they make limited use of the neurons available for sound encoding in the cochlea. To understand why, you first need to understand how natural hearing works.
In a functioning human ear, sound waves are channeled down the ear canal and set the ear drum in motion, which in turn vibrates tiny bones in the middle ear. Those bones transfer the vibrations to the inner ear’s cochlea, a snail-shaped structure about the size of a pea. Inside the fluid-filled cochlea, a membrane ripples in response to sound vibrations, and those ripples move bundles of sensory hair cells that project from the surface of that membrane. These movements trigger the hair cells to release neurotransmitters that cause an electrical signal in the neurons of the cochlear nerve. All these electrical signals encode the sound, and the signal travels up the nerve to the brain. Regardless of which sound frequency they encode, the cochlear neurons represent sound intensity by the rate and timing of their electrical signals: The firing rate can reach a few hundred hertz, and the timing can achieve submillisecond precision.
Hair cells in different parts of the cochlea respond to different frequencies of sound, with those at the base of the spiral-shaped cochlea detecting high-pitched sounds of up to about 20 kilohertz, and those at the top of the spiral detecting low-pitched sounds down to about 20 Hz. This frequency map of the cochlea is also available at the level of the neurons, which can be thought of as a spiraling array of receivers. Cochlear implants capitalize on this structure, stimulating neurons in the base of the cochlea to create the perception of a high pitch, and so on.
A commercial cochlear implant today has a microphone, processor, and transmitter that are worn on the head, as well as a receiver and electrodes that are implanted. It typically has between 12 and 24 electrodes that are inserted into the cochlea to directly stimulate the nerve at different points. But the saline fluid within the cochlea is conductive, so the current from each electrode spreads out and causes broad activation of neurons across the frequency map of the cochlea. Because the frequency selectivity of electrical stimulation is limited, the quality of artificial hearing is limited, too. The natural process of hearing, in which hair cells trigger precise points on the cochlear nerve, can be thought of as playing the piano with your fingers; cochlear implants are more equivalent to playing with your fists. Even worse, this large stimulation overlap limits the way we can stimulate the auditory nerve, as it forces us to activate only one electrode at a time.
How optogenetics works
The idea for a better way began back in 2005, when I started hearing about a new technique being pioneered in neuroscience called optogenetics. German researchers were among the first to discover light-sensitive proteins in algae that regulated the flow of ions across a cellular membrane. Then, other research groups began experimenting with taking the genes that coded for such proteins and using a harmless viral vector to insert them into neurons. The upshot was that shining a light on these genetically altered neurons could trigger them to open their voltage-gated ion channels and thus fire, or activate, allowing researchers to directly control living animals’ brains and behaviors. Since then, optogenetics has become a significant tool in neuroscience research, and clinicians are experimenting with medical applications including vision restoration and cardiac pacing.
I’ve long been interested in how sound is encoded and how this coding goes wrong in hearing impairment. It occurred to me that stimulating the cochlear nerve with light instead of electricity could provide much more precise control, because light can be tightly focused even in the cochlea’s saline environment.
We are proposing a new type of implanted medical device that will be paired with a new type of gene therapy.
If we used optogenetics to make cochlear nerve cells light sensitive, we could then precisely hit these targets with beams of low-energy light to produce much finer auditory sensations than with the electrical implant. We could theoretically have more than five times as many targets spaced throughout the cochlea, perhaps as many as 64 or 128. Sound stimuli could be electronically split up into many more discrete frequency bands, giving users a much richer experience of sound. This general idea had been taken up earlier by Claus-Peter Richter from Northwestern University, who proposed directly stimulating the auditory nerve with high-energy infrared light, though that concept wasn’t confirmed by other laboratories.
Our idea was exciting, but my collaborators and I saw a host of challenges. We were proposing a new type of implanted medical device that would be paired with a new type of gene therapy, both of which must meet the highest safety standards. We’d need to determine the best light source to use in the optogenetic system and how to transmit it to the proper spots in the cochlea. We had to find the right light-sensitive protein to use in the cochlear nerve cells, and we had to figure out how best to deliver the genes that code for those proteins to the right parts of the cochlea.
But we’ve made great progress over the years. In 2015, the European Research Council gave us a vote of confidence when it funded our “OptoHear” project, and in 2019, we spun off a company called OptoGenTech to work toward commercializing our device.
Channelrhodopsins, micro-LEDs, and fiber optics
Our early proof-of-concept experiments in mice explored both the biology and technology at play in our mission. Finding the right light-sensitive protein, or channelrhodopsin, turned out to be a long process. Many early efforts in optogenetics used channelrhodopsin-2 (ChR2) that opens an ion channel in response to blue light. We used it in a proof-of-concept experiment in mice that demonstrated that optogenetic stimulation of the auditory pathway provided better frequency selectivity than electrical stimulation did.
In our continued search for the best channelrhodopsin for our purpose, we tried a ChR2 variant called calcium translocating channelrhodopsin (CatCh) from the Max Planck Institute of Biophysics lab of Ernst Bamberg, one of the world pioneers of optogenetics. We delivered CatCh to the cochlear neurons of Mongolian gerbils using a harmless virus as a vector. We next trained the gerbils to respond to an auditory stimulus, teaching them to avoid a certain area when they heard a tone. Then we deafened the gerbils by applying a drug that kills hair cells and inserted a tiny optical cochlear implant to stimulate the light-sensitized cochlear neurons. The deaf animals responded to this light stimulation just as they had to the auditory stimulus.
The optical cochlear implant will enable people to pick out voices in a busy meeting and appreciate the subtleties of their favorite songs.
However, the use of CatCh has two problems: First, it requires blue light, which is associated with phototoxicity. When light, particularly high-energy blue light, shines directly on cells that are typically in the dark of the body’s interior, these cells can be damaged and eventually die off. The other problem with CatCh is that it’s slow to reset. At body temperature, once CatCh is activated by light, it takes about a dozen milliseconds to close the channel and be ready for the next activation. Such slow kinetics do not support the precise timing of neuron activation necessary to encode sound, which can require more than a hundred spikes per second. Many people said the kinetics of channelrhodopsins made our quest impossible—that even if we gained spectral resolution, we’d lose temporal resolution. But we took those doubts as a strong motivation to look for faster channelrhodopsins, and ones that respond to red light.
We were excited when a leader in optogenetics, Edward Boyden at MIT, discovered a faster-acting channelrhodopsin that his team called Chronos. Although it still required blue light for activation, Chronos was the fastest channelrhodopsin to date, taking about 3.6 milliseconds to close at room temperature. Even better, we found that it closed within about 1 ms at the warmer temperature of the body. However, it took some extra tricks to get Chronos working in the cochlea: We had to use powerful viral vectors and certain genetic sequences to improve the delivery of Chronos protein to the cell membrane of the cochlear neurons. With those tricks, both single neurons and the neural population responded robustly and with good temporal precision to optical stimulation at higher rates of up to about 250 Hz. So Chronos enabled us to elicit near-natural rates of neural firing, suggesting that we could have both frequency and time resolution. But we still needed to find an ultrafast channelrhodopsin that operated with longer wavelength light.
We teamed up with Bamberg to take on the challenge. The collaboration targeted Chrimson, a channelrhodopsin first described by Boyden that’s best stimulated by orange light. The first results of our engineering experiments with Chrimson were fast Chrimson (f-Chrimson) and very fast Chrimson (vf-Chrimson). We were pleased to discover that f-Chrimson enables cochlear neurons to respond to red light reliably up to stimulation rates of approximately 200 Hz. Vf-Chrimson is even faster but is less well expressed in the cells than f-Chrimson is; so far, vf-Chrimson has not shown a measurable advantage over f-Chrimson when it comes to high-frequency stimulation of cochlear neurons.
The Latest Updates from Bing News & Google News
Go deeper with Bing News on:
Optical cochlear implants
- Watch a little boy smile at hearing his parents speak with the help of cochlear implants
Nate was born with sensorineural hearing loss and relied on hearing aids. But as his hearing deteriorated, the hearing aids became less effective. To help him, he was fitted with a cochlear implant, a ...
- Boy with cochlear implants shares sweet smile after hearing parents for 1st time
An Australian mom shared a video of her son reacting to hearing his parents' voices for the first time after receiving cochlear implants.
- My daughter was born with profound hearing loss in one ear. We decided not to go through with a cochlear implant.
When her daughter was 3 months old, they found out she had profound hearing loss. They want her to decide whether she gets a cochlear implant.
- Hearing is believing: One of the biggest public tertiary health facilities has changed peoples lives with cochlear implants
The recipients of cochlear implants and their families celebrated a proud milestone at Tygerberg Hospital on Saturday, April 6. The cochlear implant is an electronic hearing device that device is made ...
- Cochlear Implant Surgery: Costs, Benefits And Risks
Cochlear implants don’t restore a person’s hearing. However, these powerful tools can help those with severe or profound hearing loss hear and process sounds better so they can stay engaged in ...
Go deeper with Google Headlines on:
Optical cochlear implants
[google_news title=”” keyword=”optical cochlear implants” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]
Go deeper with Bing News on:
Cochlear implant
- Watch a little boy smile at hearing his parents speak with the help of cochlear implants
Nate was born with sensorineural hearing loss and relied on hearing aids. But as his hearing deteriorated, the hearing aids became less effective. To help him, he was fitted with a cochlear implant, a ...
- Baha Attract System from Cochlear
The Cochlear Baha Attract System is a highly effective ... It uses a magnetic connection to attract the sound processor to the implant, sending sound to the inner ear without anything breaking ...
- New Advance in Neural Stimulation Tech Could Help Treat Neurological Disorders
This extra precision could be highly beneficial for future cochlear implants or vagus nerve stimulators, the researchers say. A research paper demonstrating the use of the prototype MagPatch on human ...
- Boy with cochlear implants shares sweet smile after hearing parents for 1st time
An Australian mom shared a video of her son reacting to hearing his parents' voices for the first time after receiving cochlear implants.
- My daughter was born with profound hearing loss in one ear. We decided not to go through with a cochlear implant.
When her daughter was 3 months old, they found out she had profound hearing loss. They want her to decide whether she gets a cochlear implant.
Go deeper with Google Headlines on:
Cochlear implant
[google_news title=”” keyword=”cochlear implant” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]